Implantable Devices
Spinal Cord Stimulators
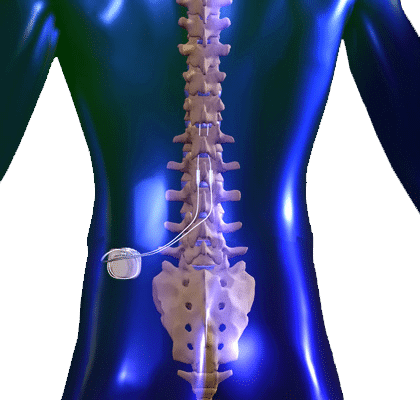
Patients who suffer from neuropathic pain, especially in the limbs, failed back surgery syndrome, complex regional pain syndrome, and sometimes chronic back pain, refractory angina pectoris, peripheral vascular disease may be candidates for spinal cord stimulation therapy. Spinal cord stimulation therapy can provide pain relief and improve limb mobility and quality of life. The device sends low level electrical signals through the electrode/lead to the posterior, sensory aspect of the spinal cord, to block the transmission of pain signals to the brain. Many patients describe the stimulation as a tingling sensation. This tingling sensation is programmed to overlap with the areas of pain, thereby blocking it. A psychological evaluation is required to assure the patient has a good understanding of the procedure and to determine if it is an appropriate option for them.
Before the spinal cord stimulation system is permanently implanted, a temporary trial is done to determine if sufficient pain relief can be achieved with this device. A temporary stimulator lead (e.g. a thin wire) is placed through the skin to the epidural space. It is connected to an external battery operated stimulator and programmed to provide stimulation (tingling) at the painful areas of the body. The leads may remain in place for approximately 3-5 days to determine if significant pain relief is achieved. Once the trial is completed, the lead is easily removed.
A permanent spinal cord system is surgically placed at least 2 weeks later to minimize risk of infection. This procedure involves inserting a new lead similarly to the trial through an incision in the skin. A battery/generator is placed under the skin in the buttock or abdominal area and connected to the lead in the back under the skin. Once the system is implanted, the stimulation system is programmed to match the painful area and the stimulation is adjusted to provide the optimal pain relief. The procedure is carried out on an outpatient basis.
There can be some pain and swelling at the incision site, which is normal and typically resolves in a few days. Short term analgesics are usually provided for pain at the incision sites. Recovery is relatively quick and patients can begin light exercise a few days following the procedure. However, lifting, bending, stretching and twisting should be avoided immediately following the implantation.
The risks of the procedure include infection, bleeding, spinal fluid leakage, paralysis, allergic reaction and nerve damage. Other risks include movement of a lead or less commonly malfunction of a lead, requiring surgical intervention to correct. Patients with spinal cord stimulators for chronic nerve pain in a limb, report a 60-80% decrease in pain following the procedure. Click here for more information.
Helpful Spinal Cord Stimulator Videos:
Intrathecal Pump Implant
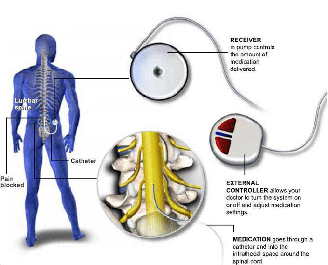
Intrathecal pump implants are indicated for patients with chronic and severe pain who have not responded to other treatment modalities. Patients who may be candidates for an intrathecal pump include those with spastic disorders, multiple sclerosis, spinal cord injuries, neuropathic pain, phantom limb pain, carpal tunnel syndrome, peripheral neuropathy, and muscle spasms.
The intrathecal pump is a device that is implanted in the body to deliver a concentrated amount of medication to the spinal fluid through a small catheter. This reduces the need for oral medications. It is commonly called the spinal morphine pump, although medications other than morphine can be used to reduce pain. The purpose of the medication is to decrease the excitatory pain signals traveling through the spinal cord and increase those signals that diminish pain, thus providing pain relief.
Before a pump can be implanted, a psychological evaluation and successful “pump trial” is required. The pump trial involves the placement of an epidural catheter, through which opioid medication is infused for a few days. If the patient experiences a significant decrease in pain during this trial, they are considered a candidate for the pump.
The implantation is a minor surgical procedure that requires a short, overnight stay. A spinal catheter is placed in the lower back and the pump is placed at the side of the abdomen. Bending, twisting, stretching and lifting should be avoided immediately after the implant.
The medication dosage is adjusted on an outpatient basis. The medication in the pump reservoir typically lasts for 1-3 months and can be refilled easily. Pumps may need to be replaced every 5-10 years if a programmable pump is used or longer if a nonprogrammable pump is used.
Intrathecal pump placement is a relatively safe procedure, although there may be some side effects from the medications that are used. Compared to oral administration of the medication, there are generally fewer side effects when it is administered in the spinal space. Side effects from opioid medications in the spinal space may include nausea, vomiting, difficulty urinating, over-sedation, muscle twitching, respiratory depression, drug reaction and confusion. However, these symptoms usually resolve within 1-2weeks and can be minimized with a low, slow titration. Additionally, local anesthetics are often placed in the intrathecal pump and may cause weakness of difficulty urinating. Clonidine, another common agent placed in the spinal space to reduce pain may cause low blood pressure, a decreased heart rate and lethargy. It is important that patients with an intrathecal pump remain in close contact with their physician to manage side effects and maximize pain relief. This communication is especially important immediately following the implantation.
As with any type of surgical intervention, there are risks associated with intrathecal pump implant. Risks include infection, bleeding, pain, allergic reactions, collections of fluid around the pump (seroma), complete or partial catheter occlusion, catheter displacement, meningitis, spinal headache, spinal fluid leaks, device failure or disconnection, and accumulation of fluids around the pump. Sometimes, surgical intervention may be necessary to correct catheter displacements or pump defects.
Intrathecal pump implant is an effective pain management procedure and 60-70% of patients report significant pain relief. Click here for more information.